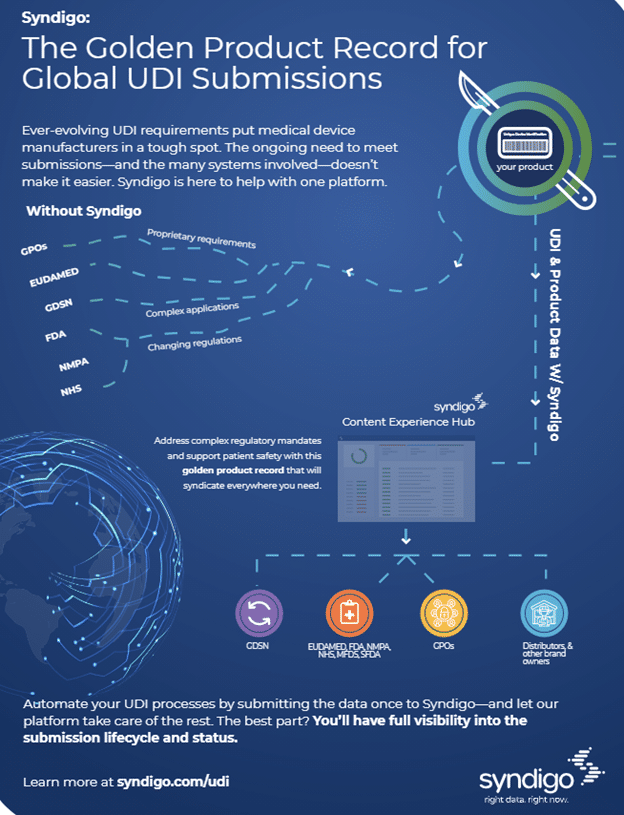
Medical device manufacturers have a lot to keep up with when it comes to submitting UDI data. Regulatory bodies all over the world have unique, demanding requirement sets—and they have a reputation for changing quite a bit.
Without a modern PIM that syndicates, these manufacturers are left scrambling to figure out the latest requirements themselves. Moreover, they need to manage each individual submission on their own and deal with tedious back-and-forth requests for updated or accurate content as circumstances evolve.
At Syndigo, we have a better option: our Syndigo Platform, the golden product record for global UDI submissions.
Click on the image below to download the infographic.